There is often a need to model the gas flow in specific systems with a temperature other than 25 C. Then we include the Energy model in the standard modeling procedure. The number of subsequent models taken into account in our analysis significantly extends the computation time. This is mainly due to the fact that the program has to solve additional equations (in our case, equations related to heat transfer). If our goal is not the temperature distribution in the analyzed domains, then we can simplify the analysis by omitting the Energy model.
 |
| How to define gas properties in Fluent |
What are the properties of gases as a function of temperature?
The main factors influencing the distribution of gas flow,
e.g. at elevated temperatures, are gas density and its viscosity. As it is
known, for most standard gases, their density decreases with increasing
temperature, while their viscosity increases. Why do we have opposite trends in
these properties of gases with increasing temperature?
As you know, gas molecules move randomly in space, often
colliding with each other. The collision causes contact between the molecules
which leads to the phenomenon of viscosity. As the temperature rises, the
movement of molecules increases (the average velocity of the particles
increases), which leads to more frequent collisions. So logically, more contact
as a result of a collision leads to an increase in the viscosity of the gas.
Effectof Temperature on Co-efficient of Viscosity in Gases - QS Study
In the case of gas density, the phenomenon of thermal expansion occurs with increasing temperature, which is also common for solids. Thermal expansion leads to the fact that the gas molecules begin to move away from each other, which means that for a constant unit of volume we have a smaller number of molecules. Fewer molecules decrease the gas density.
Whathappens with gas density when a temperature is increased? - Quora
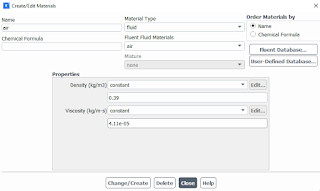 |
| Gas properties in Fluent at 700 C |
Going back to the specifics, you can turn off the energy
model while changing the properties of the gas defined in the domain. In our
case, we will only deal with the turbulence model in order to accurately
understand the gas velocity distribution in the analyzed domain.
In the Models option, we only enable the turbulent model.
Its type depends on the type of analysis we perform. For standard cases, the
default k-omega SST model is sufficient. In the properties of gases, we define
the density and viscosity of the gas corresponding to the temperature at which
we analyze our problem. Below is a link to the properties of air at different
temperatures.
Viscosityof Air, Dynamic and Kinematic | Engineers Edge | www.engineersedge.com
Also, let's not forget to define gravity from the General
tab.
Of course, by defining the properties from the level of
editing the domain material, it is also possible to analyze variants with
shifts with increased pressure, e.g. 20 bar.
Such a simplification will cause our partial equations to
converge faster and the total analysis time should be significantly shortened.
However, it should be remembered that for this type of simplifications it is
not possible to analyze temperature distributions or heat transfer coefficient
of objects.
Instead, it is an ideal approach to solving complex geometry
problems with a large number of finite elements.
In the following entries, I will also try to explain other
simplifications that can be used in specific situations.
It is worth using model simplifications while keeping in
mind the physicality of the model.
Tip
Before assuming some simplifications, analyze what factors
affect the course of the phenomena in your model.





No comments:
Post a Comment